“No element is more essential to life than carbon,” says the book Nature’s Building Blocks. The
unique characteristics of carbon enable it to bond with itself and many
other chemical elements, thus forming millions of compounds, more of
which are constantly being discovered or synthesized.
As
the examples here show, carbon atoms can also combine to form various
shapes, including chains, pyramids, rings, sheets, and tubes. Carbon
truly is a wonder element!
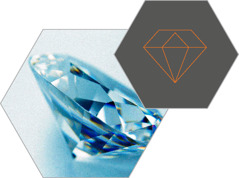
DIAMOND
Carbon
atoms form pyramids, called tetrahedrons, making the structure
extremely rigid and making diamond the hardest naturally occurring
substance known. A perfect diamond is essentially a single molecule of
carbon atoms.
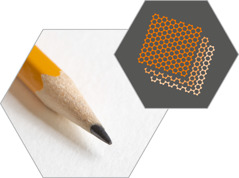
GRAPHITE
Tightly
bonded carbon atoms are set out in loosely bonded layers that can slide
away from one another like sheets of paper on a stack. Because of these
characteristics, graphite is both a fine lubricant and a key compound
in lead pencils. *
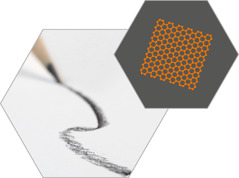
GRAPHENE
This
refers to a single layer of carbon atoms arranged in a hexagonal mesh,
or lattice. Graphene has a tensile strength many times that of steel. A
pencil trace may have small amounts of graphene in single or multiple
layers.
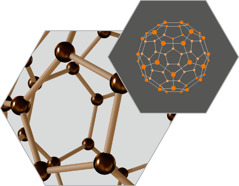
FULLERENES
These
hollow molecules of carbon come in shapes that include microscopic
balls and tubes called nanotubes. They are measured in nanometers, or
billionths of a meter.
LIVING ORGANISMS
The many cells that make up plants, animals, and humans are built on a framework of carbon